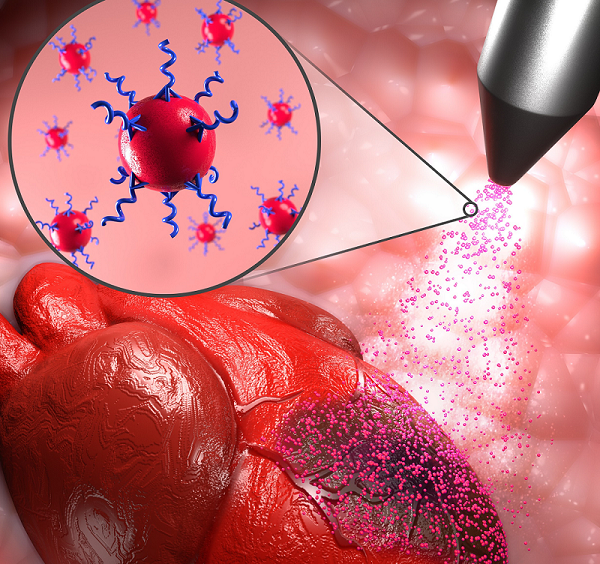
Cutting-edge research co-led by uOttawa Faculty of Medicine Associate Professor Dr. Emilio I. Alarcón could eventually impact millions of lives with peptide-based hydrogels that will close skin wounds, deliver therapeutics to damaged heart muscle, as well as reshape and heal injured corneas.
“We are using peptides to fabricate therapeutic solutions. The team is drawing inspiration from nature to develop simple solutions for wound closure and tissue repair,” says Dr. Alarcón, a scientist and director at the BioEngineering and Therapeutic Solutions (BEaTS) group at the University of Ottawa Heart Institute whose innovative research work is focused on developing new materials with capabilities for tissue regeneration.

Peptides are molecules in living organisms and hydrogels are a water-based material with a gelatinous texture that have proven useful in therapeutics.
The approach used in the study – just published in Advanced Functional Materials and co-led by Dr. Erik Suuronen & Dr. Marc Ruel – is unique. Most hydrogels explored in tissue engineering are animal-derived and protein-based materials, but the biomaterial created by the collaborative team is supercharged by engineered peptides. This makes it more clinically translatable.

Dr. Ruel, a full professor in the uOttawa Faculty of Medicine’s Department of Cellular and Molecular Medicine and the endowed chair of research in the Division of Cardiac Surgery at the University of Ottawa Heart Institute, says the study’s insights could be a game changer.
"Despite millennia of evolution, the human response to wound healing still remains imperfect,” Dr. Ruel says. “We see maladapted scarring in everything from skin incisions to eye injuries, to heart repair after a myocardial infarction. Drs. Alarcón, Suuronen, and the rest of our team have focused on this problem for almost two decades. The publication by Dr. Alarcón in Advanced Functional Materials reveals a novel way to make wound healing, organ healing, and even basic scarring after surgery much more therapeutically modulatable and, therefore, optimizable for human health.”
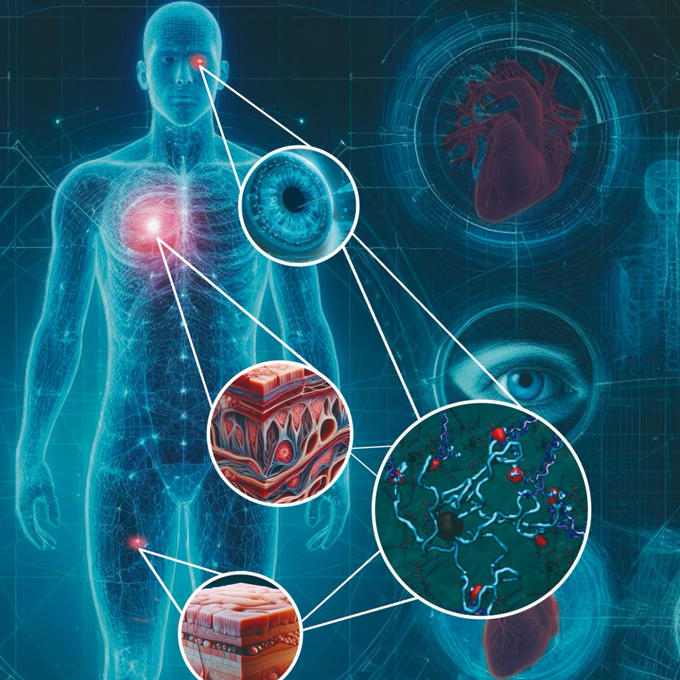
Indeed, the ability to modulate the peptide-based biomaterial is key. The uOttawa-led team’s hydrogels are designed to be customizable, making the durable material adaptable for use in a surprising range of tissues. Essentially, the two-component recipe could be adjusted to ramp up adhesivity or dial down other components depending on the part of the body needing repair.
“We were in fact very surprised by the range of applications our materials can achieve,” says Dr. Alarcón. “Our technology offers an integrated solution that is customizable depending on the targeted tissue.”
Dr. Alarcón says that not only does the study’s data suggest that the therapeutic action of the biomimetic hydrogels are highly effective, but its application is far simpler and cost-effective than other regenerative approaches.
The materials are engineered in a low-cost and scalable manner – hugely important qualities for any number of major biomedical applications. The team also devised a rapid-screening system that allowed them to significantly slash the design costs and testing timespans.
“This significant reduction in cost and time not only makes our material more economically viable but also accelerates its potential for clinical use,” Dr. Alarcón says.
What are next steps for the talent-rich research team? They will conduct large animal tests in preparation for tests in human subjects. So far, heart and skin tests were conducted with rodents, and the cornea work was done ex vivo.
Part of the work for this study was funded by the uOttawa Faculty of Medicine’s “Path to Patenting & Pre-Commercialization” (3P), an innovation-focused approach to provide our community’s top-flight researchers with the assistance needed to bring their most promising breakthroughs to the wider world.
Support the Faculty of Medicine today!
Use the “Other designation” field on our online donation form to support the Faculty of Medicine Scholarship for Graduate Research