Dr. Robert Ben of uOttawa’s Department of Chemistry leads multi-million-dollar initiative that thwarts ice growth to preserve samples for future use
The makeup of a cold-water fish has inspired a University of Ottawa scientist to develop a method for freezing stem cells and tissue without the risk of “freezer burn.”
Dr. Robert Ben, a professor in the Department of Chemistry, is one half of the brainchild behind the development of ice recrystallization inhibitors, which are small organic molecules that halt ice growth in order to better preserve biological material used in the fields of cell therapy and regenerative medicine.
The idea to thwart this by-product of freezing “crystalized” in Dr. Ben after learning about the teleost fish, a species that can survive in sub-zero environments because their bodies use anti-freeze proteins to inhibit the growth of ice crystals.
Robert Ben, who specializes in synthetic organic and medicinal chemistry at uOttawa, developed the technology alongside Dr. Jason Acker of the University of Alberta at PanTHERA CryoSolutions. This private company co-owned by the pair will receive $4 million in private funding over the next two years to develop this technology and others like it, including one to preserve COVID-19 testing materials and RNA-based vaccines.
How did this come about?
“We have been freezing cells and tissues for some time now, in order to develop cell therapies to treat a wide range of diseases and we’ve been using cryoprotective agents, such as dimethyl sulfoxide or glycerol, since the 1950s to try to prevent the cells from dying in the freezing and thawing process.
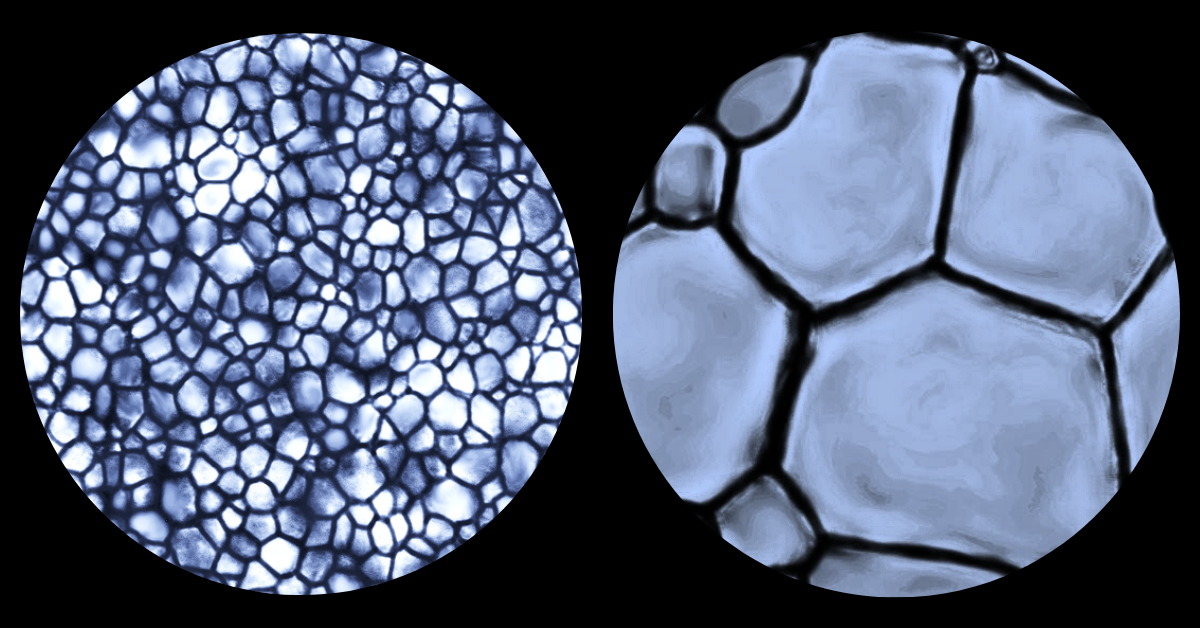
“The problem with current cryoprotectants is that cell recovery is kind of hit and miss. We might freeze 100,000 cells, but only 25,000 will survive and be viable for research or clinical applications. That’s because up to 80 percent of the cellular damage that happens during freezing is due to the uncontrolled growth of ice. Since current cryoprotectant solutions don’t address this problem, our returns, measured in cell recovery and function, are quite dismal.”
Shouldn’t freezing samples be more efficient?
“Ice growth, or the process of ice recrystallization, is an inevitable side-effect of freezing something and over time, and with temperature fluctuations, ice crystals become larger and larger and cause a lot of disruption in cell membranes, which in turn damages or kills the cells.
“Think of freezer burn; if you’ve ever tasted ice cream after it’s been sitting in your freezer for a while — I’m sure we all have — the product looks and tastes different from when it was brand new. That’s because those ice crystals are changing the structure of that material, and along with that goes taste and everything else.”
Why are large ice crystals damaging to cells?
"Small ice crystals are innocuous but large ice crystals damage cell membranes easily. These small crystals are like grains of sand on a Caribbean beach which are so small that they mould to your body and you can lay comfortably on the beach for an entire day. Now, let’s say those grains of sand were replaced by gravel or pebbles. That’s a lot less comfortable. Our cryopreservation technology prevents ice crystals from growing (and thus remain small) during freezing and thawing ensuring the survival of cells."
What will the impact of this be?
“Cells, tissue, organs — and potentially vaccines and other biological materials — can be kept at warmer temperatures, making it easier to store these products and ship them to remote locations. The lower the temperature, the slower the recrystallization process. That’s why some therapies such as vaccines need to be stored at very cold temperatures so they can be preserved for longer. For example, the Pfizer COVID-19 vaccine must be kept at -70 degrees Celsius to keep the ice crystals from growing too large and damaging the product.”
With modern cell therapies and regenerative medicine techniques on the rise, it must be important to preserve materials that make such medical advances possible.
“Our molecules are unique because, unlike conventional cryoprotectants, they prevent all that cellular damage caused by ice. In the end, we recover more cells, they’re healthier and more functional. There is nothing else like it out there.”