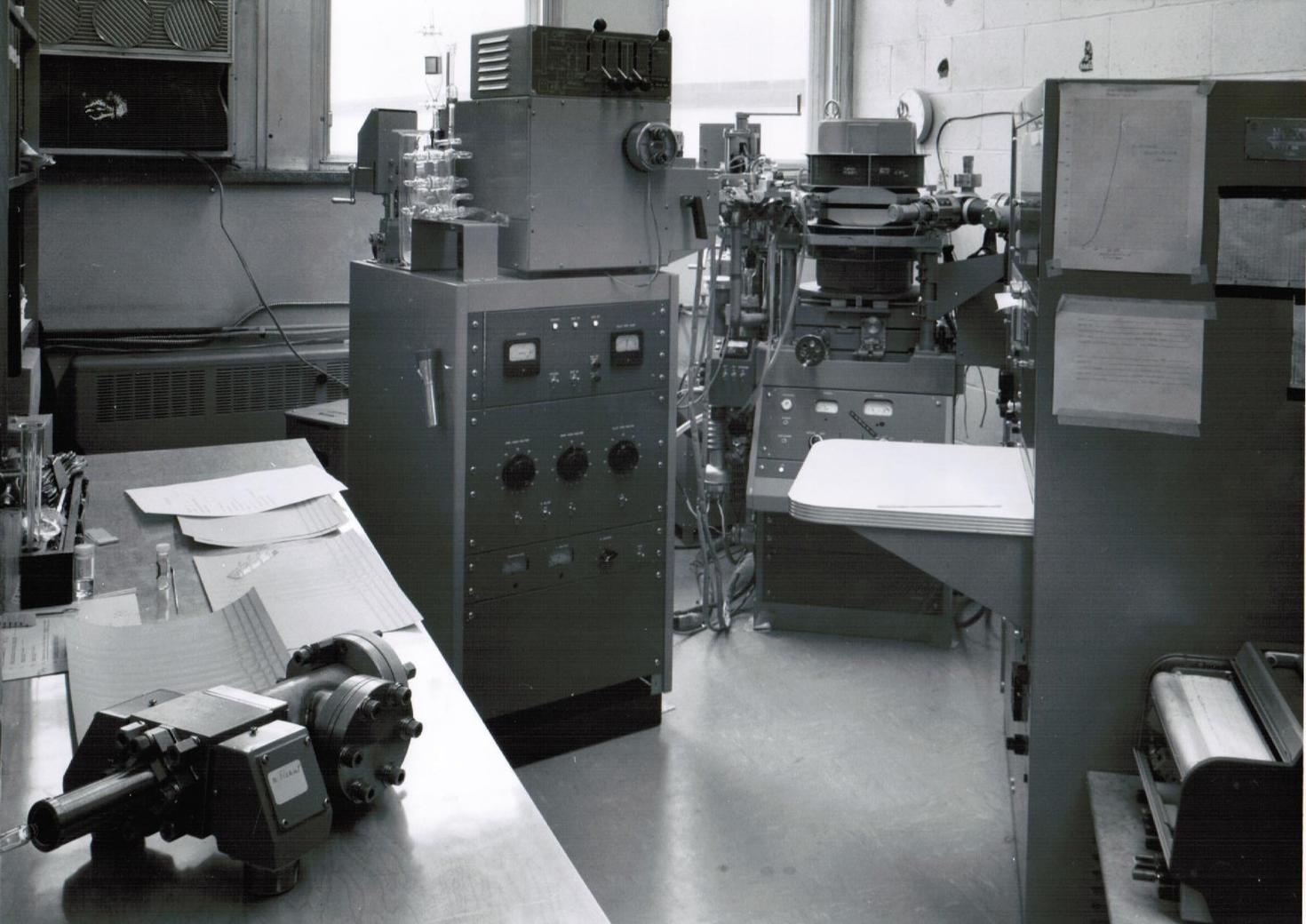
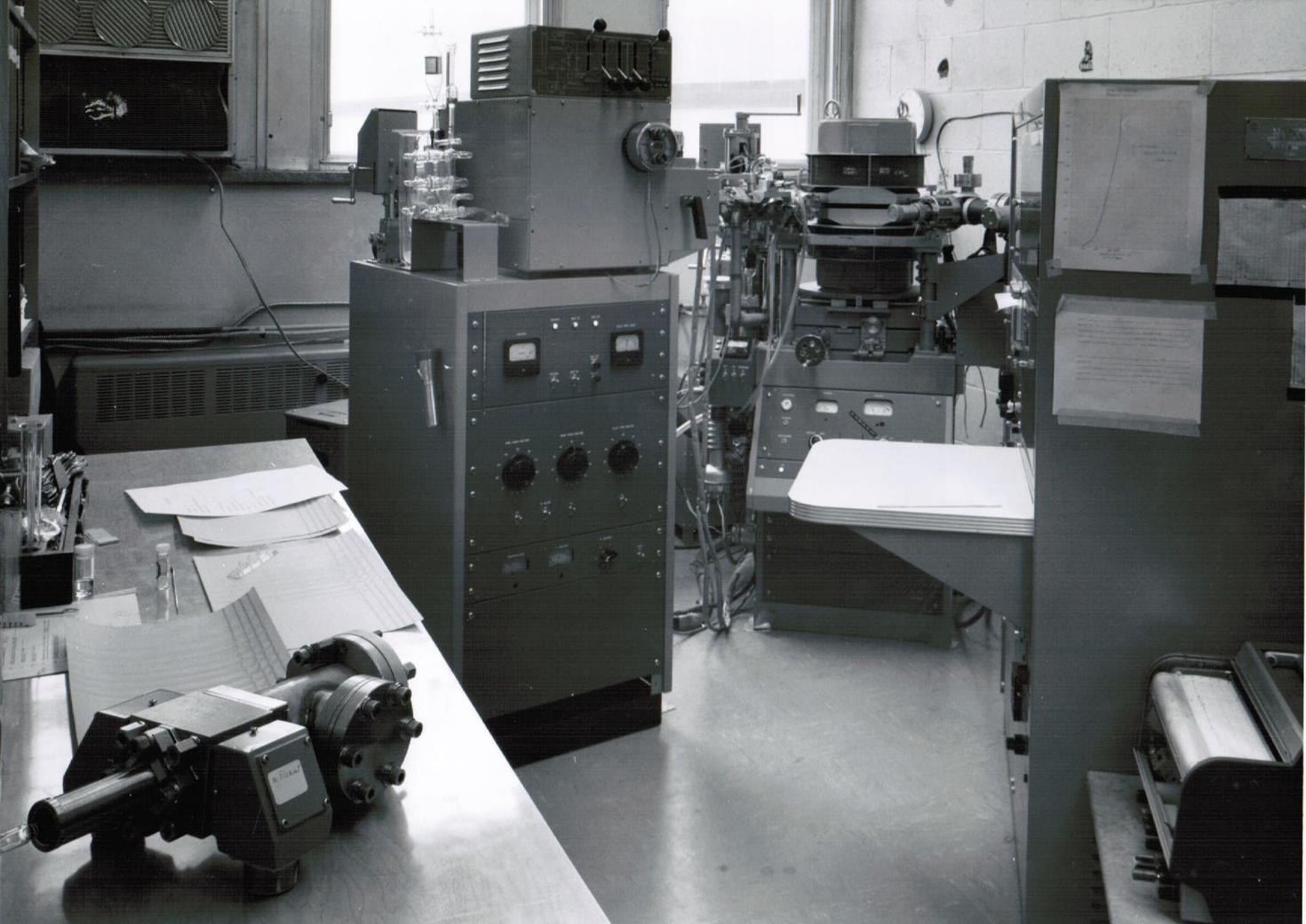
The laboratory began in the spring of 1964, when the Department was awarded a grant of $56,000 by the NRC, (which then was the research grant-giving agency in Canada) for the purchase of its first ever analytical mass spectrometer (MS). The grant was announced at a staff meeting by the Chair, Professor Keith Laidler, but no-one showed much interest in taking on the task of selecting, maintaining and running the instrument and providing mass spectra for all comers. In the event, John Holmes volunteered to take the responsibility, and with Fred Lossing he visited the Perkin-Elmer factory in Norwalk, Connecticut. Fred liked the look of the RMU-6D and so the two orders were placed. (It should be recalled that Fred Lossing was a pioneer in gas phase ion chemistry, his speciality being the accurate measurement of the ionization energies of small molecules and especially, thermally generated free radicals. He was searching for a routine MS to keep the organic chemists at the NRC content)
History of the facility
Mass Spectrometry in the Chemistry Department; a Brief History 1964-2000

The RMU-6D was installed in November of that year. It was a good, single-focusing, magnetic sector instrument; all the electronics depended upon vacuum tubes and the ion acceleration potential was 3000V. With vacuum tubes one had to beware of residual potentials when carrying out routine maintenance because the related condensers retained their charge and so should be grounded before touching. The high vacuum was achieved by means of liquid nitrogen (in those days provided free, by the NRC) cold traps attached to oil diffusion pumps. The electron-impact (IE) ion source was simple to service, although glass-blowing skills were required for filament replacing. One special addition was a pair of ion repeller plates in the source; these allowed the source residence time to be altered as well as for optimising the ion beam intensity, a very good feature signally lacking in all subsequent instrumentation. Gas, liquid and solid samples could be introduced without difficulty. On a very good day, the mass resolution was sufficient to just separate CO+•, HCNH+, N2+• and C2H4+•. Its mass range was to ca 1000 a.m.u. The mass spectra (ms) were recorded by a “Visicorder”, in which four spot galvanometers (sensitivities 1:3:10:30) projected their beam onto UV light-sensitive paper, that developed on exposure to the room lights.
Holmes’ research speciality up to then was gas-phase free radical and atom reactions in pyrolytic and photochemical reactions and so the interpretation of mass spectra was not of much scholarly interest. Note that the first serious book about the interpretation (and rationalisation) of mass spectra, by Fred McLafferty, did not appear until 1966, and the first scientific journals devoted to mass spectrometry, only appeared in 1968---“The International Journal of Mass Spectrometry and Ion Physics” and “Organic Mass Spectrometry”----following proposals that summer at an international meeting in Berlin.
The interpretation of mass spectra was then based on reaction mechanisms taken from classical (neutral) organic chemistry, and the complex fragmentation schemes that appeared in MS publications were ingenious but wholly imaginary. The fundamental problem was that the myriad stable unconventional ion structures that we accept today (2015) were then undiscovered, not least because their neutral counterparts were either unstable or unknown and so inaccessible to direct experiments. Much of the research in the MS laboratory has been to discover methods for determining both the energetics and structures of gas phase ions.
That the subject certainly required some important new ideas is well exemplified by two early observations:- ionized 2,3,4-trimethlypentane yields intense C4H9+ and C4H7+ fragment ions, and ionized benzene loses a CH3• radical---inexplicable by conventional reaction mechanisms.
In 1968, quite by happenstance (routine mass spectra of samples from the UK), Holmes noted that the molecular ions of many dicarboxylic acids lost CO2, but in a manner dependent upon the position and orientation of the carboxyl groups. Deuterium labelling was easily performed by exchange with D2O and some novel fragmentation mechanisms were unravelled. Note that inter alia, benzoic acid (OD) was shown to lose •OH and •OD radicals in a ratio of 2:1, showing that the carboxyl H and (by further labelling) the two ortho-H atoms became equilibrated before the ion dissociated. Even more complex, the t-butyl cation, (CH3)313C+ fragments with loss of 13CH4 as well as CH4, in the statistical ratio of 1:3.
Much of this research involved the examination of metastable ion peaks, those diffuse signals that appear at non-integral mass in simple sector instrument recordings. These diffuse signals arise from ions that dissociate in the field-free regions of the MS. Their shapes arise from the release of internal energy of the ions into translational degrees of freedom. These complex shapes (profiles) reflect the potential energy surface(s) over which the ions fragment and these in turn, relate to ion structures. A noteworthy example is the castellated peak that accompanies the loss of H2 from C3H5+ cations, showing the generation of the isomeric C3H3+ species, the propargyl and cyclopropenyl cations.

An EB double focussing, high resolution MS, an AEI MS902S instrument, was obtained form an NSERC grant of $130,000 in 1972. The chief motive for its acquisition was to provide accurate mass determinations for the organic chemists, to show that their carefully isolated products (sometimes) had the correct molecular formula. It was installed in Marion Hall (through the lab windows) onto a steel and cement slab mounted upon inflatable rubber doughnuts to provide a vibration-free mount, upon which the instrument could be rocked gently to and fro, with a natural frequency of ca 0.5s-1. Its initial resolving power was a stunning 180,000, as demonstrated by the happy installation engineer, Ernie Mills. However that quickly deteriorated to ca 30,000 as normal wear and tear took effect. In this instrument the electronics were also still vacuum-tube controlled and the electric sector potential was maintained by large dry batteries and again, when servicing the apparatus, great care was required to avoid any (residual) accelerating potential of 8000V. This fine MS also allowed the separation of finely resolved metastable ion peaks from the normal ms, and research with Hans Terlouw (then on a year’s leave from his home University in Utrecht), allowed us to clearly distinguish between the isomeric C2H4O+• ions, acetaldehyde (A), vinyl alcohol (V) and oxirane, as molecular and as product ions in a ms. (Thus for example, ionised 2-propanol was shown to yield a mixture of isobaric A and V ions via two CH4 losses, competing 1,2 eliminations). The study of metastable ion properties occupied several busy years. For a progress review, see Org. Mass Spectrom. 15, 383-396, (1980). This happy collaboration with JKT continued for the next 40 years and we have shared in some 70 joint publications.
In 1980 Fred Lossing retired from the NRC and we transported his precious electron mono-chromator to our lab on the back of an open truck, and it was installed for him in a small adjoining room. Fred interacted closely with the research students and visiting scientists and many happy hours were spent discussing the intricacies of ion structures and their thermochemistry, and also curing the ills of the world. Fred stayed with us for the next 15 years and the very stimulating collaboration produced some 35 joint publications.
In 1982, a proposal to the NSERC for a MS Centre at the University of Ottawa was fully funded ($680,000) and the lab quickly expanded to house a VG 7070 (EB) medium resolution analytical MS and a ZAB-2F, a reversed geometry, BE, instrument of exceptional versatility. An Infrastructure Grant and support for service work from both Ottawa Universities (at $24,000 each, p.a.) and the NRC, ensured a flying start to a very productive period of research and a considerable expansion of the lab’s analytical facilities, including the later move to the larger laboratory in the D’Iorio building in 1992. Very useful additional monetary support came in the late 80’s and early 90’s for an additional small analytical research laboratory, funded by Glaxo, for developing methods for identifying synthetic intermediates in pharmaceuticals, for environmental analyses, and a network of small commercial users and University connections was established. The general analytical workload was ca 2500-3000 samples per annum.
The research years from 1982-1997, produced unique methods for studying the neutral products of ion fragmentation; the neutralisation of ion beams with Xe and a range of metal atom vapours created novel neutral radicals and molecules---some stable, some transient; the emission of radiation from excited ion beams; charge reversal mass spectra (positive to negative and vice versa), and more. An early major breakthrough came from the study of new ions of unconventional structure, the so-called distonic ions, which are stable isomers of conventionally structured ions. It began with Hans Terlouw discovering (in Utrecht) that the ion produced by the loss of CH2O from ionized propane-1,3-diol has the structure CH2CH2OH2+• (an ionised ethene/water complex) and is therefore not an expected ionised C2H6O molecule (Org. Mass Spectrom.16, 326-332, (1981) ). This opened the flood gates to the discovery of a myriad such stable ions involving N, O, S and halogen atoms; for example, the stable ion CH2OH2+• which has a lower enthalpy of formation than the conventional CH3OH+• species. For the radiation experiments in 1992 the ZAB-2F was given a third sector with a quartz window and a long track below the beam path, enabling experiments to be mounted to intercept the ion beam. The apparatus was also wholly refurbished, thanks to a $485,000 grant from NSERC, increasing the versatility of the instrument yet again.
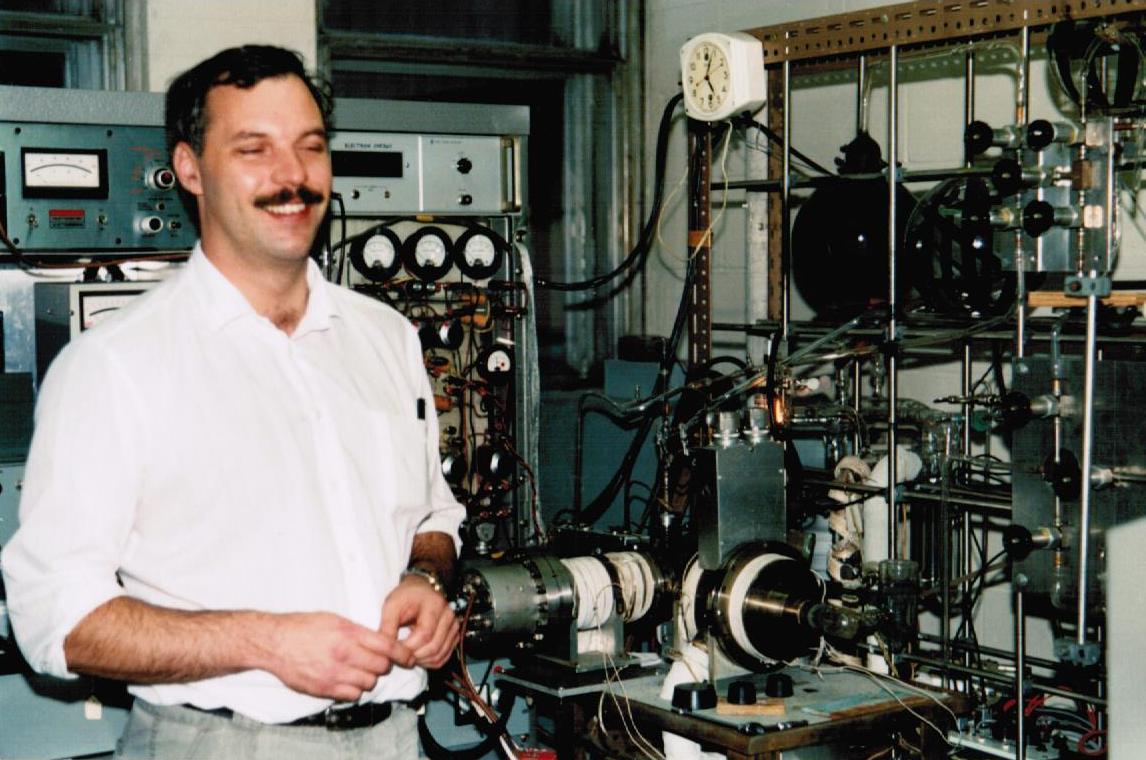
In the MS laboratory, so much has depended on the permanent staff, without whom the enterprise would certainly have failed. These include Pierre Coulombe, Jake Blair, John Kraus, Clem Kazakoff, Sander Mommers and Sharon Curtis. Sander Mommers in particular, has been responsible for the construction and maintenance of all the vital accessories for the ZAB, without which much of the exciting research could not have been accomplished. Other special collaborators and friends include Dr. M. Dakubu (University of Ghana in Accra), Dr. Neil Isaacs (University of Reading. UK), Dr. Robin Rye (Concordia University), Dr. A. D. Osborne (Queen Mary University, London UK), Prof. Leo Radom (University of Sydney), Dr P. C. Burgers (Erasmus Medical Centre, Rotterdam), Prof. J. H. Bowie (University of Adelaide), and special mention for Prof. Paul Mayer and Dr Christiane Aubry, both ex-graduate students, whose support and enthusiasm have been beyond price.
Some more of this story can be found in “Mass Spectrometry and the Pleasures of Science”, Org. Mass Spectrom. 28, 1388-1394, (1993), which explains how many of the projects evolved. For the early years and history of mass spectrometry, review articles by Sy Meyerson should be inspected (Analytical Chem. 66, 960-964, (1994) and Org. Mass Spectrom. 21, 197-208, (1986)). John Holmes.

Sharon Curtis replaced him in 2013 as the facility manager. Paul Mayer, Sharon and Sander continued the mass spectrometry tradition at University of Ottawa, until they were joined by Maxim Berezovski in 2014. Maxim brought with him two Thermo Fisher Orbitrap and with these the Biological facility was born.
In 2022, after 40 years of service, Sander Mommers retired.
The Facility continues to serve the UOttawa Community and More.

Contact us
Chemical facility
Sharon Curtis
sharon.curtis@live.ca
D'Iorio Hall - Room 124
Ottawa, ON K1N 6N5
Tel: 613-562-5800 ext. 604
Biological facility
Zoran Minic
zminic@uottawa.ca
Marion Hall - Room 002
Ottawa, ON K1N 1A2
Tel: 613-562-5800 ext. 1626